J. Biosci. Agric. Res. | Volume 20, Issue 01, 1664-1670| https://doi.org/10.18801/jbar.200119.202
Article type: Research article|Received: 14.01.2019, Revised: 04.02.2019, Date of Publication: 14 April 2019.
Article type: Research article|Received: 14.01.2019, Revised: 04.02.2019, Date of Publication: 14 April 2019.
Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan
Shahab Habib 1, Luqman Amrao 1, Muhammad Zeshan Ahmed 1, Maaz Ahmad 1, Shanam Naz 1, Muhammad Ahmad Zeshan 4, Hafiz Muhammad Asadullah 2, Abdul Manan Hamza 3 and Salman Ghuffar 1
1 Dept. of Plant Pathology, University of Agriculture Faisalabad, Pakistan
2 Pulses Research Program, NARC Islamabad, Pakistan
3 Dept. of Entomology, PMAS-Arid Agriculture University, Rawalpinidi, Pakistan
4 Dept. of Plant Pathology, University of Sargodha, Sargodha, Pakistan.
1 Dept. of Plant Pathology, University of Agriculture Faisalabad, Pakistan
2 Pulses Research Program, NARC Islamabad, Pakistan
3 Dept. of Entomology, PMAS-Arid Agriculture University, Rawalpinidi, Pakistan
4 Dept. of Plant Pathology, University of Sargodha, Sargodha, Pakistan.
Abstract
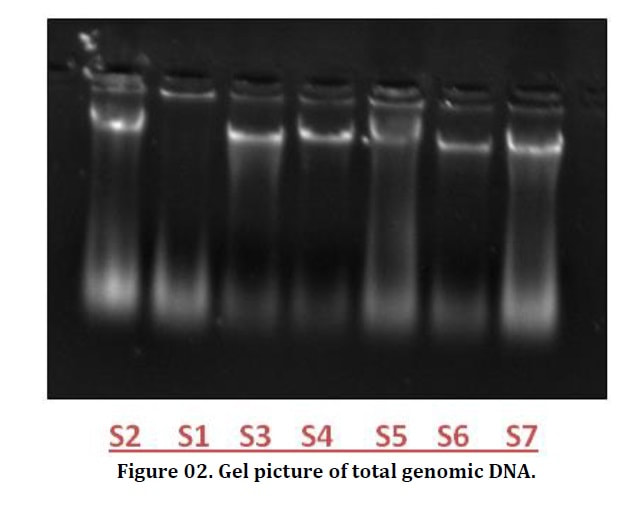
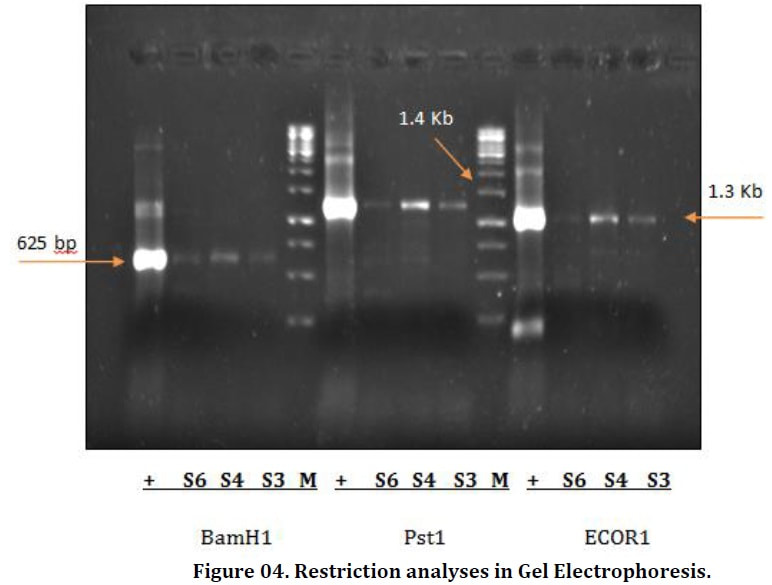
Shoe flower (Hibiscus rosa-sinensis L.) is an ornamental plant belongs to the family Malvaceae. Due to its natural beauty and medicinal properties it is mainly grown in regions with tropical and subtropical environment. Shoe flower is known to infect by many diseases. Among all diseases Hibiscus leaf curl disease is caused by the begomovirus transmitted through Bemisia tabaci is very common and also an alternative host of cotton leaf curl disease due to the similar symptomology. In present study the identification of begomovirus in shoe flower was analyzed by using PCR-RFLP (Polymerase chain reaction- Restriction fragment length polymorphism). For this purpose, survey was conducted from seven different locations in university of agriculture Faisalabad. Plants were observed with virus symptoms of Hibiscus leaf curl disease such as upward curling of leaves, darkening and thickening of veins, stunting of plant size as well as leaf enation. For further molecular studies, DNA was isolated from all infected samples by using CTAB method and confirmed by Gel electrophoresis. PCR reaction amplified three DNA samples having beta satellite component, labeled (S3, S4 and S6) at 1.4 kbp. According to the (PCR-RFLP) analysis of three amplified samples, In BamH1 restriction enzyme, all the samples were restricted into 625 bp. In second restriction enzyme Pst1, DNA samples were restricted into 1.4 kbp. While in the case of ECOR1, fragment was restricted into 1.3 kbp.
Key Words: Shoe flower, DNA extraction, PCR, PFLP, Beta satellite and Begomovirus
Shoe flower (Hibiscus rosa-sinensis L.) is an ornamental plant belongs to the family Malvaceae. Due to its natural beauty and medicinal properties it is mainly grown in regions with tropical and subtropical environment. Shoe flower is known to infect by many diseases. Among all diseases Hibiscus leaf curl disease is caused by the begomovirus transmitted through Bemisia tabaci is very common and also an alternative host of cotton leaf curl disease due to the similar symptomology. In present study the identification of begomovirus in shoe flower was analyzed by using PCR-RFLP (Polymerase chain reaction- Restriction fragment length polymorphism). For this purpose, survey was conducted from seven different locations in university of agriculture Faisalabad. Plants were observed with virus symptoms of Hibiscus leaf curl disease such as upward curling of leaves, darkening and thickening of veins, stunting of plant size as well as leaf enation. For further molecular studies, DNA was isolated from all infected samples by using CTAB method and confirmed by Gel electrophoresis. PCR reaction amplified three DNA samples having beta satellite component, labeled (S3, S4 and S6) at 1.4 kbp. According to the (PCR-RFLP) analysis of three amplified samples, In BamH1 restriction enzyme, all the samples were restricted into 625 bp. In second restriction enzyme Pst1, DNA samples were restricted into 1.4 kbp. While in the case of ECOR1, fragment was restricted into 1.3 kbp.
Key Words: Shoe flower, DNA extraction, PCR, PFLP, Beta satellite and Begomovirus
Article Full-Text PDF
| 202.01.20.19_identification_of_beta_satellite_component_in__hibiscus_rosa-sinensis_l.__through_pcr-rflp_from_faisalabad_pakistan.pdf | |
| File Size: | 792 kb |
| File Type: | |
Article Metrics
|
Share This Article
|
|
Article Citations
MLA
Habib, et al. “Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan.” Journal of Bioscience and Agriculture Research 20(01) (2019): 1664-1670.
APA
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. (2019). Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research, 20(01), 1664-1670.
Chicago
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. “Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan.” Journal of Bioscience and Agriculture Research 20(01) (2019): 1664-1670.
Harvard
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. 2019. Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research, 20(01), pp. 1664-1670.
Vancouver
Habib S, Amrao L, Ahmed MZ, Ahmad M, Naz S, Zeshan MA, Asadullah HM, Hamza AM and Ghuffar S. Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research. 2019 April 20(01): 1664-1670.
Habib, et al. “Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan.” Journal of Bioscience and Agriculture Research 20(01) (2019): 1664-1670.
APA
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. (2019). Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research, 20(01), 1664-1670.
Chicago
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. “Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan.” Journal of Bioscience and Agriculture Research 20(01) (2019): 1664-1670.
Harvard
Habib, S., Amrao, L., Ahmed, M. Z., Ahmad, M., Naz, S., Zeshan, M. A., Asadullah, H. M., Hamza, A. M. and Ghuffar, S. 2019. Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research, 20(01), pp. 1664-1670.
Vancouver
Habib S, Amrao L, Ahmed MZ, Ahmad M, Naz S, Zeshan MA, Asadullah HM, Hamza AM and Ghuffar S. Identification of beta satellite component in (Hibiscus rosa-sinensis L.) through PCR-RFLP from Faisalabad, Pakistan. Journal of Bioscience and Agriculture Research. 2019 April 20(01): 1664-1670.
References
- Bertaccini, A. L., Carraro, D., Davies, D. A., Laimer, M., Camara, M., Martini, M., Paltrinieri, S. and Seemüller, E. (2000). Micropropagation of a collection of phytoplasma strains in periwinkle and other host plants, p. 101. In: 13thInternational Congress of IOM, July 14-19, Fukuoka, Japan.
- Briddon, R. W. and Markham, P. G. (2000). Cotton leaf curls virus disease. Virus Research 71, 151-159. https://doi.org/10.1016/S0168-1702(00)00195-7
- Briddon, R. W., Bull, S. E. Amin, I., Idris, A. M., Mansoor, S. I., Bedford, D., Dhawan, P., Rishi, N., Siwatch, S. S., Abdel-Salam, A. M., Brown, J. K., Zafar, Y. and Markham, P. G. (2002). Diversity of DNA β: a satellite molecule associated with some monopartite begomoviruses. Virol. 312, 106-121. https://doi.org/10.1016/S0042-6822(03)00200-9
- Briddon, R. W., Mansoor, S. I., Bedford, D., Pinner, M. S., Saunders, K., Stanley, J., Zafar, Y., Malik, K. A. and Markham, P. G. (2003). Identification of DNA components required for induction of cotton leaf curl disease. Virol. 285, 234-243. https://doi.org/10.1006/viro.2001.0949
- Brown, J. K., Fauquet, C. M., Briddon, R. W., Zerbini, F. M., Moriones, E. and Navas-Castillo, J. (2012). Family Geminiviridae. In Virus Taxonomy 9th Report of the International Committee on Taxonomy of Viruses, pp. 351–373. Edited by A. M. Q. King, M. J. Adams, E. B. Carstens and E. J. Lefkowitz. London, UK: Elsevier Academic Press.
- Chomczynski, P. and Sacchi, N. (2006). The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on Nature Protocols 1, 581-585.
- Doyle, J. J. and Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus 12, 13-15.
- Harrison, B. D., Liu, Y. L., Khalid, S., Hameed, S., Otim-Nape, G. W. and Robinson, D. J. (1997). Detection and relationships of cotton leaf curl virus and allied whitefly-transmitted geminiviruses occurring in Pakistan. Annals of Applied Biol. 130, 61–75. https://doi.org/10.1111/j.1744-7348.1997.tb05783.x
- Idris, A. M., Shahid, M. S., Briddon, R. W., Khan, A. J., Zhu, J. K. and Brown, J. K. (2011). An unusual alpha satellite associated with monopartite begomoviruses attenuates symptoms and reduces beta satellite accumulation. J. Gen. Virol. 92, 706–717. https://doi.org/10.1099/vir.0.025288-0
- Islam, M. M., Adhikary, S. K., Gain, P., Rahman, M. M. and Siddique, M. N. A. (2004). Effect of plant growth regulators on callus induction and plant regeneration in another culture of rice. Pakistan J. Biol. Sci., 7, 331-334. https://doi.org/10.3923/pjbs.2004.331.334
- Lolita, M., Dolores, M., Gonzales, C. and Magdalita, P. M. (2014). Isolation and identification of a begomovirus associated with the leaf curl disease of Gumamela (Hibiscus rosa-sinensis) in the Philippine. Asian journal of agriculture and food sciences 2, 566-576.
- Mali, V.R. (1980). Studies on leaf curl disease of China rose in Marathwada. Indian Journal of Mycol and Plant Pathol. 9, 119.
- Mansoor, S., Khan, S. H., Bashir, A., Saeed, M., Zafar, Y., Malik, K. A., Briddon, R. W., Stanley, J. and Markham, P. G. (1999). Identification of a novel circular single-stranded DNA associated with cotton leaf curl disease in Pakistan. Virol. 259, 190-199. https://doi.org/10.1006/viro.1999.9766
- Qazi, J., Amin, I., Mansoor, S., Iqbal, J. and Briddon, R. W. (2007). Contribution of the satellite encoded gene βC1 to cotton leaf curl disease symptoms. Virus Research128, 135-139. https://doi.org/10.1016/j.virusres.2007.04.002
- Rajeshwar, R. R., Reddy, V. C., Maruthi, M., Colvin, N. J., Seal, S. E. and Muniyappa, V. (2005). Host range, vector relationships and sequence comparison of a begomovirus infecting hibiscus in India. Ann Appl Biol. 147, 15–25. https://doi.org/10.1111/j.1744-7348.2005.00005.x
- Relman, D. A., Schmidt, T. M., Gajadhar, A., Sogin, M., Cross, J., Yoder, K., Sethabutr, O. and Echeverria, P. (1996). Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J. Infect. Dis. 173, 440-445. https://doi.org/10.1093/infdis/173.2.440
- Rummel, D. J. (2005). Botanical beauty book compendium of cosmetic uses. C and E Publishing, Inc. Quezon City. Philippines. p. 433.
- Sharma, S. and Sultana, S. (2004). Effect of Hibiscus rosa-sinensis extract on hyper proliferation and oxidative damage caused by benzoyl peroxide and ultraviolet radiation in mouse skin. Basic Clin Pharmacol Toxicol 95(5), 220-225. https://doi.org/10.1111/j.1742-7843.2004.pto950504.x
- Sunter, G., Hartitz, M. D., Hormuzdi, S. G., Brough, C. L. and Bisaro, D. M. (1990). Genetic analysis of tomato golden mosaic virus: ORF AL2 is required for coat protein accumulation while ORF AL3 is necessary for efficient DNA replication. Virol. 179, 69-77. https://doi.org/10.1016/0042-6822(90)90275-V
- Sultana, J., Sutlana, N., Siddique, M. N. A., Islam, A. K. M. A., Hossain, M. M. and Hossain, T. (2011). In vitro bulb production in Hippeastrum (Hippeastrum hybridum). Journal of Central European Agriculture, 11(4), 469-474. http://dx.doi.org/10.5513/jcea.v11i4.849
- Siddique, M. N. A., Sultana, J., Sultana, N. and Hossain, M. (2007). Effect of planting dates on growth and flowering of Hippeastrum (Hippeastrum hybridum). Int. J. Sustain. Crop. Prod. 2(5), 12-14.
- Siddique, M. N. A., Sultana, N., Haque, M. A., Hossain, M. M. and Ahmed, J. U. (2006). Effects of twin scale size and hormones on in vitro propagation of Hippeastrum (Hippeastrum hybridum). Plant Tissue Culture and Biotechnology, 16(2), 105-110. http://dx.doi.org/10.3329/ptcb.v16i2.1111
- Varma, A. and Malathi, V. G. (2003). Emerging geminivirus problems: A serious threat to crop production. Annals of Applied Biology 142, 145-164. https://doi.org/10.1111/j.1744-7348.2003.tb00240.x
- Vasudeva, R. S., Varma, P. M. and Capoor, S. P. (1953). Some whitefly (Bemisia tabaci) transmitted viruses in India. In: 6th International Congress of Microbiology, Rome 5, 520–521.
© 2019 The Authors. This article is freely available for anyone to read, share, download, print, permitted for unrestricted use and build upon, provided that the original author(s) and publisher are given due credit. All Published articles are distributed under the Creative Commons Attribution 4.0 International License.
Journal of Bioscience and Agriculture Research EISSN 2312-7945.